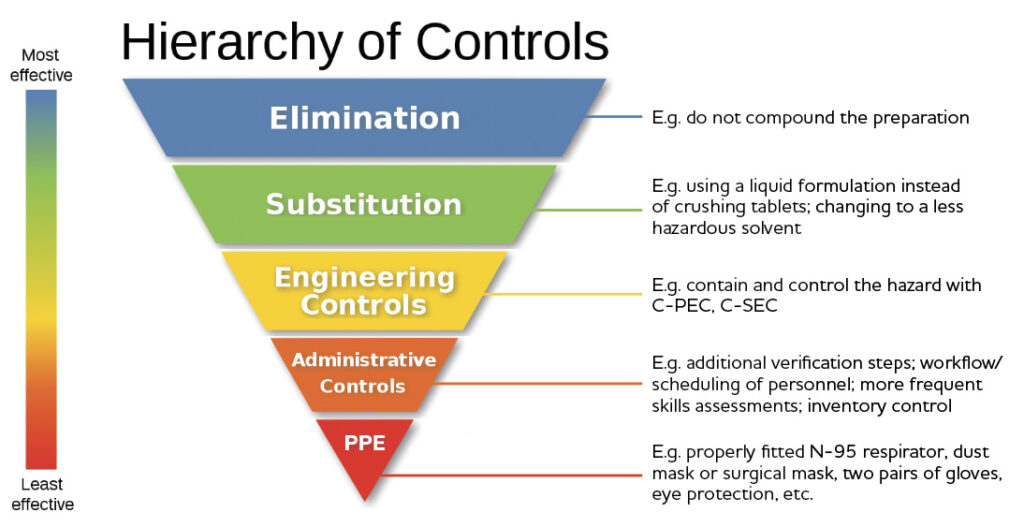
Refer to Sections 9.1, 9.1.1, 9.2.1, 9.2.2 and 9.3 of the NAPRA Guidance Document for Pharmacy Compounding of Non-sterile Preparations.
Compounding of hazardous non-sterile preparations should occur in a separate room specifically dedicated for this purpose.
The level of requirements needed (i.e., B or C) depends more on the risk(s) posed by the hazardous product than on the complexity of the preparations. Drugs listed in NIOSH† Group 1 (antineoplastic/cytotoxic) require handling in the greater precautions provided by Level C (e.g., a closed-off room under negative pressure, filtered air exhausted to the outside, etc.) to avoid contaminating the environment and to further protect personnel. Similarly, compounding with drugs categorized by WHMIS‡ as a health hazard because they are very irritating to the respiratory tract, skin and/or mucous membranes should also take place in a Level C room.
If it is not possible to have a dedicated room for hazardous compounding, at an absolute minimum there should be assurances that the area is meticulously cleaned before non-hazardous preparations are compounded. To prevent any risk of cross-contamination, the compounding area, equipment and accessories must be deactivated, decontaminated and cleaned as described in Section 9, immediately after compounding with the hazardous materials.
Because of the difficulty of removing hazardous product contamination, the surfaces of ceilings, walls, floors, fixtures, shelving, counters and cabinets in the non-sterile compounding area should be smooth, impermeable, free from cracks and crevices, and made of non-shedding material.
As part of the pharmacy’s quality assurance program, personnel must be trained to perform these tasks and their work routinely assessed to ensure compliance with policies and procedures.
†National Institute for Occupational Safety and Health
‡Workplace Hazardous Materials Information System